Introduction:
Medical advances in the care of patients with sickle cell disease (SCD) and other hemoglobinopathies have allowed for the focus of care to shift and incorporate long-term quality of life metrics, such as fertility. Concerns for reproductive health in patients with SCD stem from the underlying disease itself as well as from medical therapies that are offered such as hydroxyurea, chronic red blood cell transfusions, and stem cell transplant (SCT). Iron deposition from chronic transfusions in other tissues is known to result in end organ damage and lifelong consequences. While little is known about iron overload and fertility, one study noted testicular iron deposition on MRI in pubertal males with transfusion-dependent beta-thalassemia; results were notable for lower sperm concentrations and a lower percentage of sperm with normal morphology, indicating that iron overload likely has an impact on fertility for patients. Iron deposition has not been studied in the pre-pubertal hemoglobinopathy population. Fertility counseling in this patient population does not often occur until patients are considering SCT as a curative therapy option, as the conditioning regimens for SCT are gonadotoxic. Without a good understanding of the impact of chronic transfusions on fertility for patients with hemoglobinopathies, it is difficult to determine the optimal time to offer fertility preservation to families. We aim to assess iron overload on testicular tissue to gain a better understanding of the impact that medical management and chronic transfusions can have on fertility for a patient with SCD.
Methods:
Our study is a cross-sectional evaluation of patients with SCD who underwent testicular tissue cryopreservation (TTC) as a part of a multicenter research protocol through the University of Pittsburgh Medical Center (UPMC) prior to SCT. As part of the TTC protocol, each patient provided a small piece of tissue for research, with the remaining cryopreserved for patient use. All samples were evaluated for the presence of germ cells.
For this study, demographics, treatment data, imaging results, and labs pertinent to hormonal profile and iron status were obtained. All samples from patients with SCD and thalassemia will undergo additional pathology review to evaluate for the presence of germ cells and iron.
At the time of this abstract, the tissue for two patients had been analyzed.
Results:
Banked research tissue from UPMC includes 77 patients with SCD and 21 patients with beta thalassemia major. Of the 77 SCD patient samples, 42 were found to have germ cells, 34 have not yet been analyzed and 1 had no germ cells present. Of the 21 samples from patients with beta thalassemia major, 17 were found to have germ cells, 3 were not yet analyzed and 1 had no germ cells present.
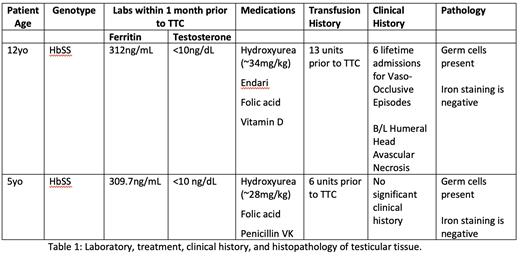
To date, we have obtained pathology review on two patients, both with Hb SS genotype and were on the maximum tolerated dose of hydroxyurea prior to TTC. Patient 1 had been on hydroxyurea for 7 years and had 13 units of blood transfusions, and Patient 2 on hydroxyurea for 4 years and 6units of blood transfusions prior to TTC. Both patients were found to have normal testicular tissue histopathology with presence of germ cells and without evidence of iron on staining (Table 1).
Conclusions:
Based on our preliminary data it can be noted that patients without significant iron burden, based on low ferritin and a limited transfusion history may not have a significant impact on their testicular histopathology with their iron staining being negative. We will expand our patient cohort to include patients who have a severe disease course requiring more blood transfusions to determine whether this is associated with increased iron deposition in their testicular tissue and/or reduced germ cell numbers. This information will allow teams to optimize the timing of fertility discussions and possible preservation in this patient population, even outside of curative treatment.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal